Upcoming Events!
Past Events

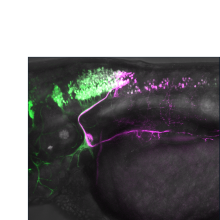
Abstract: During development, the central nervous system establishes precise connections with the...
Abstract: During development, the central nervous system establishes precise connections with the body to coordinate organ function. A crucial component of communication between the brain and body is the vagus nerve (cranial nerve X), which innervates multiple organ systems including the heart, lungs and digestive tract to regulate blood pressure, heart rate, respiration and digestion. Despite this important role, the molecular mechanisms guiding the vagus nerve to these organ targets during development remain unknown. We have developed the zebrafish embryo as a powerful model for interrogating vagus nerve development, taking advantage of its optical clarity and genetic accessibility. Using a novel photoconversion-based retrograde axon tracing approach we show that vagal motor neurons (mXns) that project to different organs (e.g. gallbladder, stomach, intestines) are spatially segregated within the hindbrain vagus nucleus. We hypothesize that these distinct mXn "target groups" have distinct molecular identities that guide axon targeting. To test this hypothesis, we have generated a developmental scRNAseq atlas focused on cranial motor neurons and have validated the spatially restricted expression of transcription factors and cell-surface molecules within the vagus motor nucleus. We have generated genetic tools to correlate gene expression with target groups, and performing a reverse mutagenesis screen to test the role of these candidates in topographic map formation, revealing preliminary mXn identity phenotypes. We have also observed that mXn axons contact specific subsets of enteric neurons (ENS) during motor axon pathfinding and have begun testing the role of these contacts in guiding topographic motor targeting.
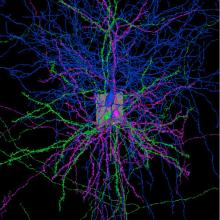

Synopsis: My lab studies the circuit mechanisms that enable oxytocin (OXT)-producing neurons in the...
Synopsis: My lab studies the circuit mechanisms that enable oxytocin (OXT)-producing neurons in the vertebrate hypothalamus to control diverse behaviors. Working in larval zebrafish, we have found that OXT neuron activity elicited by noxious stimuli helps drive the acute, defensive response to physical threats through temporally sustained but spatially precise neuropeptide release within a brainstem premotor network. A parallel project demonstrates that OXT plays an evolutionarily conserved role in affiliative social behavior and socially-reinforced learning in the miniature fish, Danionella cerebrum. In this talk I'll discuss both those stories and their implications for an emerging picture of OXT's integrated physiological and behavioral roles.

Neural manifolds are a language for describing the structure of population activity in neural...
Neural manifolds are a language for describing the structure of population activity in neural populations, which is intrinsically shaped by the structure of the information the population encodes. Tools from the mathematical field of algebraic topology allow us to detect and characterize this intrinsic structure within individual neural populations without reference to external correlates. However, as experimental tools increasingly allow us to study multiple brain regions at once, it has become possible to ask how such structured information flows and transforms as it moves through the brain. As these topological tools do not rely on prior knowledge or hypotheses about what is encoded, they are ideally suited to frame and answer such questions. In this talk, we will discuss recent work developing computational tools for studying how structure in neural manifolds align (or fails to), and forthcoming work on how we can use these tools to study learning in biological neural systems. No prior understanding of topology or topological methods in neuroscience will be assumed.

Abstract: Neuroplasticity is an important feature of respiratory control and critical for life, as...
Abstract: Neuroplasticity is an important feature of respiratory control and critical for life, as this allows for adjustments to breathing to fit our metabolic needs across development, disease, injury, and aging. Nicotine is a highly addictive recreational drug that modulates neuronal excitability and plasticity, and the impact of in utero nicotine exposure on the development and function of central networks that control breathing have been extensively studied. Conversely, despite the widespread use of tobacco and nicotine products, we know little about how chronic nicotine exposure impacts breathing control in adulthood. This seminar will highlight some of the ways the Wollman lab studies nicotine-mediated plasticity of respiratory control by giving an overview of past and current research projects including 1) work in neonatal rats showing that in utero nicotine exposure modulates fast-synaptic transmission, indicating a potential mechanism for Sudden Infant Death Syndrome, and 2) Dr. Wollman’s current NIH funded research, which is the first to show the detrimental effects of acute nicotine withdrawal on respiratory chemoreflex control in adult rats.

Dr. Carly S. Cox is currently a Science Policy Analyst for the Institute of Defense Analyses, a...
Dr. Carly S. Cox is currently a Science Policy Analyst for the Institute of Defense Analyses, a federally-funded group that advises Congress on policy pertaining to biotechnology and biosecurity. Carly earned her Bachelor's degree in Biochemistry and Molecular Biology at the University of Georgia, then earned her Master's as well as her Doctorate degree in Molecular, Cellular, and Developmental Biology at Yale University. She started out her career as an intern for Connecticut Congresswoman Rosa DeLauro in her 14th term, and served as a point of contact between DeLauro and constituents as well as government agencies. Since then, she has worked as a science policy advocate for several non-profits, including Research! America, a medical and health research advocacy group aimed at educating and lobbying for policy that supports public health, and the Council on Strategic Risks, a non-partisan group focused on advocating for U.S. biosecurity and biodefense. Carly has lived all over the east coast and currently lives in Washington D.C.
Food and beverages will be provided

ION Fall Rotation Talks
Tuesday, December 10, 2024
2:30 PM - 3:30 PM in 150 Columbia Hall
- 2:30 PM Joe...
ION Fall Rotation Talks
Tuesday, December 10, 2024
2:30 PM - 3:30 PM in 150 Columbia Hall
- 2:30 PM Joe Wargo - Niell Lab (ION)
- 2:45 PM Danielle Alonzo - Huxtable (Human Phys)
- 3:00 PM Jeremy Guenza-Marcus - McCormick (ION)
- 3:15 PM Brooke Frohock - Gardner (Knight Campus)
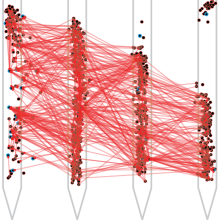
Organisms continually tune their perceptual systems to the features they encounter in their...
Organisms continually tune their perceptual systems to the features they encounter in their environment. We have studied how this experience reorganizes the synaptic connectivity of neurons in the olfactory cortex of the mouse. We developed an approach to measure synaptic connectivity in vivo, training a deep convolutional network to reliably identify monosynaptic connections from the spike-time cross-correlograms of 4.4 million single-unit pairs. This revealed that excitatory piriform neurons that respond similarly to each other are more likely to be connected. We asked whether this like-to-like connectivity was modified by experience but found no effect. Instead, we found a pronounced effect of experience on the connectivity of inhibitory interneurons. Following repeated encounters with a set of odorants, inhibitory neurons that responded differentially to these stimuli both received and formed a high degree of synaptic connections with the cortical network. The experience-dependent organization of inhibitory neuron connectivity was independent of the tuning of either their pre- or their postsynaptic partners. These results suggest the existence of a cell-intrinsic, non-Hebbian plasticity mechanism that depends only on the odor tuning of the inhibitory interneuron. A computational model of this plasticity mechanism predicts that it increases the dimensionality of the entire network’s responses to familiar stimuli, thereby enhancing their discriminability. We confirmed that this network-level property is present in physiological measurements, which showed increased dimensionality and separability of the evoked responses to familiar versus novel odorants. Thus a simple, cell-intrinsic plasticity mechanism acting on inhibitory interneurons may implement a key component of perceptual learning: enhancing an organism’s discrimination of the features particular its environment. [Work with Andrew Fink and Samuel Muscinelli]
